Particle size analysis for the quality control of active pharmaceutical ingredients (API)
Baclofen belongs to the group of drugs of muscle relaxants and is being prescribed as a mono product. Is it primarily used for the treatment of spastic movement disorder, e.g. in instances of multiple sclerosis or spinal cord injury.
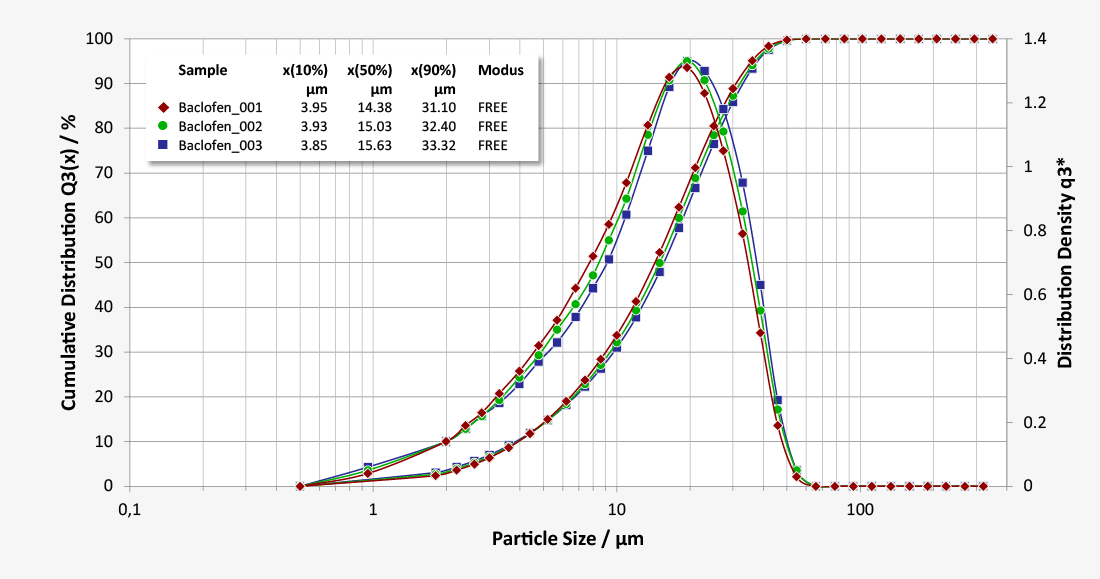
Baclofen is a white mostly odourless crystalline powder, slightly soluble in water and insoluble in chloroform or hexane. The solid dosage form as tablets contains 10 mg or 20 mg of the active pharmaceutical ingredient (API). As particle size plays a major part in the release of active ingredients, a mean particle diameter of below 20 µm is targeted during the manufacturing of tablets. This ensures a controlled release of ingredients. Laser diffraction can help to control the micronisation of the ingredients to its desired size at a high rate of reproducibility, even for small sample amounts.
- Due to safe handling and solubility, the particle size analysis is performed in hexane
- Small liquid volume during measurement
- Ultrasonic dispersion in measuring system prior to measurement
- Easy and complete cleaning of measuring system
- Expectation of high measurement frequency
Download application note for detailed information
Are you interested in additional content? Please register an account. After confirming the registration link brochures, application notes and other documents on particle measurement will be available for download.
Application strengths
- Measuring system for small sample amounts with integrated ultrasound transmitter
- Low consumption of approximately 50 ml hexane for one measurement
- Easy sample dosing with spatula, inserted directly into cuvette
- Small sample amounts due to long laser path length through sample (20 mm) in comparison to standard flow through cuvettes (2 mm)
- Easy removal and cleaning of cuvette
- High throughput rate of samples due to usage of multiple cuvettes
- Good reproducibility despite of small sample amount
Customer benefits
- Fast and easy control of micronisation
- Good reproducibility of method
- Good system-to-system-comparability allows easy transfer of methods to different production sites
- Safe control of approval relevant particle size independent of user and location