Particle size analysis in pharmaceutical manufacturing during grinding process
The pharmaceutical industry places the highest requirements on quality and reliability in the manufacturing and application of pharmaceutically active substances and excipients. The objective is not only to safely handle these substances during production and to protect the operating personnel, but also to ensure faultless product quality using an appropriate quality concept in the product and process development, the so-called Quality by Design (QbD). Part of this concept is the Process Analytical Technology (PAT) initiative of the US Food and Drug Administration (USFDA), who monitor and manage the production processes from the intermediate products to the completion of the final product using in-process control measures. This requires the timely measurement of critical process parameters (CPP) which influence the critical quality attributes (CQA) for source materials and in-process materials. This is achieved using suitable systems for analysing and controlling the production processes.
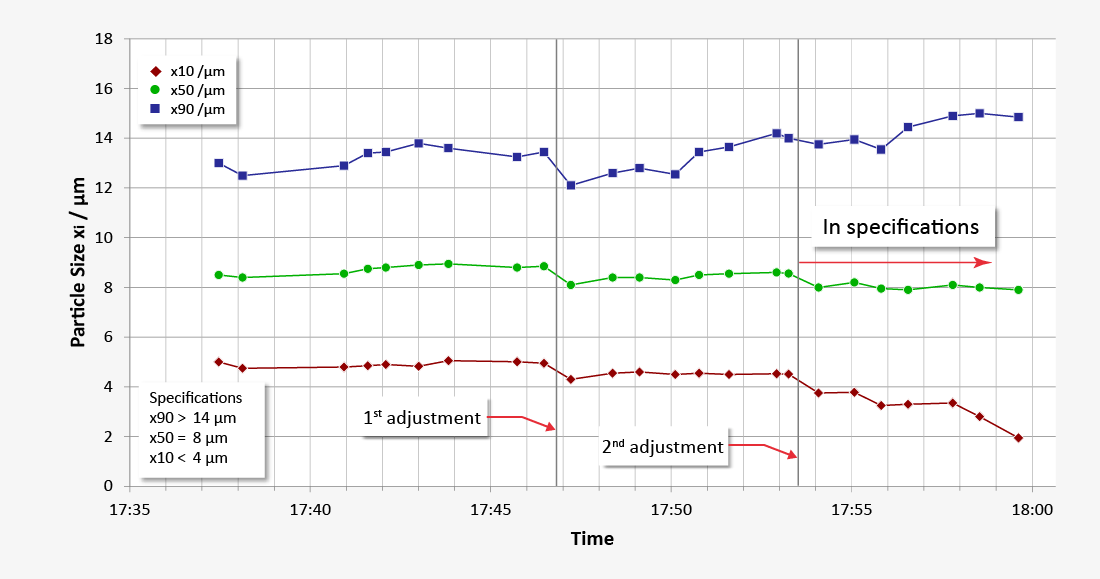
A common process step in the manufacturing of finely dispersed active ingredient powder for solid dosage forms is air jet grinding in the kilo lab. Without suitable online analysis the grinding process would have to be interrupted on a regular basis in order to analyse the grinding progress in the lab using individual, manually removed samples. The grinding process is suspended until the analysis results are available.
Online particle size analysis, however, provides real-time monitoring of the grinding level – without interrupting the grinding process and while shielding the active substance in the sense of containment. Using appropriate sampling systems such as TWISTER or probes makes it possible to both retrieve minimal, but nevertheless representative product quantities from the process and discharge them on the MYTOS laser diffraction sensor. The online monitoring provides reliable measurement results for particle size distribution in real time. Grinding parameters such as form, quantity and classifier speed can thus be optimised in operation. The active ingredient remains in a closed system through the entire process. This means that contamination can be avoided within the scope of the analysis.
- Ensuring perfect quality in the manufacturing of active pharmaceutical ingredients and excipients
- In-process control of particle size for managing the grinding process
- Real-time analyses with small amounts of active ingredient
- Continuous monitoring of the target particle size
- Avoiding contamination
- Protection of the operating personnel in case of potent active substances
Download application note for detailed information
Are you interested in additional content? Please register an account. After confirming the registration link brochures, application notes and other documents on particle measurement will be available for download.
Application strengths
- Representative sampling of < 0.5 g per measurement | With TWISTER
- Reproducible results with high sensitivity
- RODOS dry dispersion and autofocus detector in MYTOS
- Fast and easy cleaning of TWISTER & MYTOS
- GMP-compliant design | Suitable for clean-in-place (CIP)
- Applicable in potentially explosive environments | ATEX
Customer benefits
- Particle size distribution for milling control in real time
- Avoiding faulty batches or scrap material
- Seamless quality control of the whole production campaign
- No interruption of the grinding process for sampling
- Combined process and quality control
- Contamination-free working