Importance of particle sizing for active pharmaceutical ingredients in semi-solid dosage form as suppositories
Medication is being used in a semi-solid dosage as suppositories if an oral application of active pharmaceutical ingredients (API) through mouth and gastrointestinal tract is limited, e.g. if the patient has difficulties swallowing, is unconscious or unwilling. Furthermore the insertion of API into the rectum can help to avoid the First-pass-effect (conversion of APIs within the liver). It can also achieve longer lasting depot effects or local effects within the rectum.
Hard fat (e.g. coconut oil or palm kernel oil) is the base of producing suppositories containing lipids. It melts at body temperature and releases the incorporated ingredients. There are also preparation methods using a water-soluble process. Typical applications for rectal suppositories are gastroenteritis, migraine or, especially for children, the treatment of fever and pain.
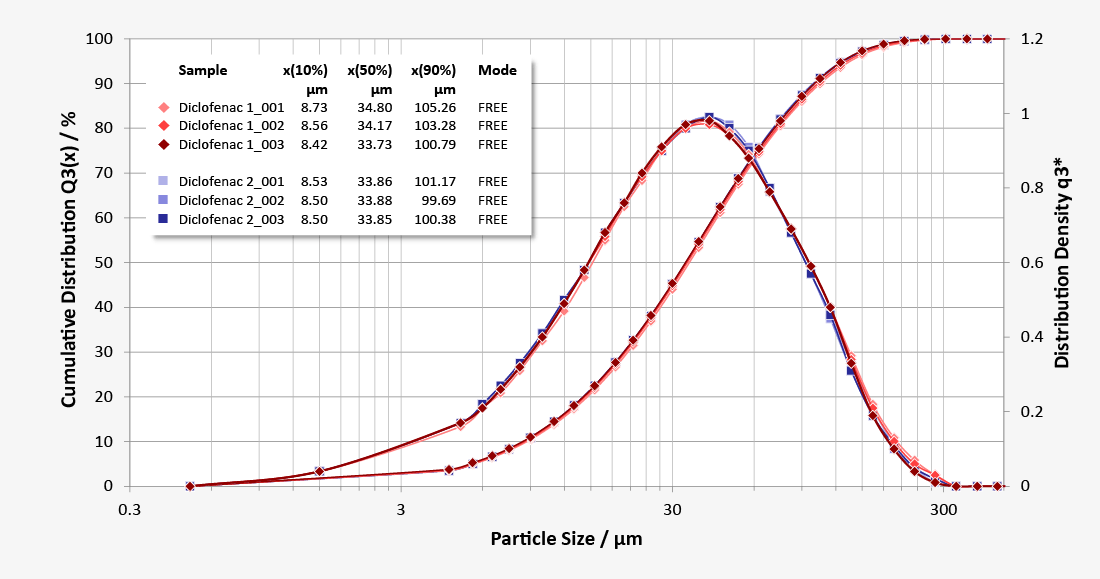
Pharmaceutical regulations require control of the quality parameters of a dosage form in its final state. In order to control the particle size, the whole suppository is melted in an individually tempered wet measuring unit to release its active components. The modular laser diffraction system HELOS with the wet dispersing unit QUIXEL offers the perfect combination for this measuring task. Equipped with centrifugal pump, quick draining and heating option, QUIXEL is the first choice. It meets the requirements of routine standard analysis based upon Standard Operating Procedures (SOPs) within quality control as well as complex and individual measuring tasks in the course of developing semi-solid dosage forms.
- Measuring particle size of suppository’s active ingredient in molten state
- Utilises a dispersion liquid close to hard fat for melting and measuring
- Flexible analysis volume for different dosage forms and active ingredients
- Simple handling and fast measurement
- Easy, time-saving cleaning
Download application notes for detailed information
Forgot your password?
Please enter your username or email address. Instructions for resetting the password will be immediately emailed to you.
Are you interested in additional content? Please register an account. After confirming the registration link brochures, application notes and other documents on particle measurement will be available for download.
Application strengths
- Fast, repeatable and reproducible measurement in tempered paraffin oil
- Easy, time-saving cleaning
- Flexible analysis volume up to 1 liter to measure a complete suppository
- Optional heating module for continuous temperature control of dispersing liquid